Corporate News

08 Mar, 2022
Today on this International Women's Day we'd like to put the spotlight on some very impressive and talented Women in Perigord. This important date on the calendar marks the liberation of women and gives them equal rights. 'Here's to strong women, may we know them, may we be them, may we raise them.'

20 May, 2021
Join us and your industry peers for our latest Webinar "How the power of a connected AMS and automated proofreading solution will improve the efficiencies of your process and increase your speed to market" on-demand now where experts from Dr. Pfleger Arzneimittel GmbH, Perigord and Schlafender Hase will share their experience and demonstrate how having an AMS system with integrated digital comparison functionality will increase your speed to market, reduce the risk of product recall, and provide a full end-to-end audit trail of all approvals.

10 May, 2021
As industry leaders and key sponsors at the Pharma Packaging & Labelling V-Forum on 26-27 May 2021 , we look forward to meeting with other pharma packaging supply chain professionals and reviewing the challenges and opportunities that the ever changing marketing dynamics are creating for our industry. As the pharmaceutical sector evolves, we will explore a number of key challenges that continue to beset the industry and add complexity in the artwork and labelling processes. Our COO, Global Software Division, Suzanne Ivory and Global Business Development Director, Mark Ennis will be in attendance. We hope you can join Suzanne who will be speaking on day 2 on these topics, providing a look into the future and outlining the solutions available to help pharmaceutical organisations navigate the challenges ahead.

05 May, 2021
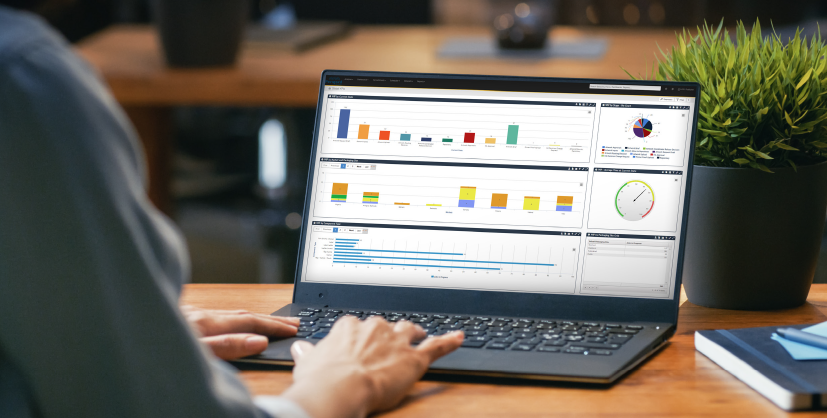
Dublin, Ireland – May 05, 2021: Deep search capabilities and extensive integration options amongst suite of enhancements for life science-specific artwork management system GLAMS 5 Perigord announces the release of GLAMS 5.0 , the latest update to its industry-leading AMS platform. Developed to the specific needs of the Life Science industry, this latest release boasts an expanded suite of both new and improved features, as well as greatly improved performance. Perigord Data’s Chief Operations Officer, Suzanne Ivory explained, “GLAMS 5’s new deep search capabilities break down potential impediments to getting products to market, and allows users to create deep and wide-ranging searches of all their artwork data, including querying active text on any uploaded PDFs, making it possible to gather a huge amount of rich, context-filled information quickly, easily, and efficiently . ” With its history of innovation and close cooperation with some of the world’s biggest pharmaceutical companies, Perigord’s development team has added several new features to GLAMS to greatly increase the usability and productivity of the platform for its users. These include: Advanced search capabilities Enhanced built-in proofing and reporting tools Configurable document stamping Extensive integration enhancements Multilingual versions Performance improvements “ Listening to our customers and leveraging our own experience, we have spent several months refining the GLAMS platform , ” explained Paul Leamy, Group CTO and Head of Product Innovation at Perigord Data . “ We have added new functionality and increased the processing speed across our product suite. Not only do the new features offer our clients a more holistic experience when managing their artwork, GLAMS 5.0 will greatly improve the speed, usability, scalability, and efficiency of the artwork and labelling process across all phases of the packaging and labelling supply chain for our clients .” GLAMS is the only artwork management system built specifically for the exacting requirements of the Life Science industry and the release of GLAMS 5 is a further step toward cementing Perigord’s reputation as the go-to AMS solution provider in the sector. “ It is Perigord’s extensive expertise and experience that enables us to develop solutions that streamline the approval process and other aspects of artwork and labelling workflows, essentially removing the bottlenecks that delay the packaging development process ,” commented Perigord Data’s Director of Client Services, Sam Cole . Introducing GLAMS 5’s new features: Advanced search capabilities – Leveraging the power of OCR technology, the scope of GLAMS 5’s capabilities have increased massively, allowing users to search the text of supporting or approval documents. Searches have also been made faster and more intuitive, enhancing clients’ capacity to access artwork tasks quicker, create complex, ad hoc queries conveniently, and deliver even greater efficiency to the process. Enhancements to integrated proofing and reporting tools – Clients can seamlessly integrate with the newest versions of InformaIT, GlobalVision, TVT and other leading comparison tools on the market as well as enjoying improvements to the GLAMS 3D visualisation functionality. These enhancements also provide customers with the added ability to autosave reports from third-party applications directly into GLAMS jobs. Configurable document stamping – This extension to the existing GLAMS stamping functionality allows multiple approver stamps to be shown in a table on a separate page inserted into an approved artwork PDF. This improvement also allows for the inclusion of pagination on stamping activities, making quality assurance processes more configurable, more efficient, and providing greater traceability in offline, printed artwork and graphic files. Approver stamps are clearer and more easily located and viewed. Extensive integration upgrades – Updates to the GLAMS API layer deliver improved connectivity to external systems and make GLAMS the most integration-ready AMS on the market. This will enable users to send and receive data from a variety of sources including ERP systems like SAP or Oracle, as well as Regulatory Information, Documents Management, Change Control, and PLM systems, unleashing a huge range of digital capabilities. Multilingual versions – The GLAMS user interface can now be tailored to clients’ language preferences. Pre-configured, multi-lingual options now include Japanese and Mandarin, with the facility to add additional language packs as required. Performance improvements – Perigord’s continued focus on under-the-hood investment has led to a raft of improvements that speed up the loading of data in grids and drop-down values, allowing for faster job scheduling, quicker search replies and more efficient data export. These performance improvements deliver quicker response times, enhancing the user experience. “ With the new functionality and system improvements included in GLAMS 5.0, I am confident that GLAMS is the best Life Science artwork management system on the market ,” said Perigord Data’s COO, Suzanne Ivory . “ The improvements incorporated into GLAMS 5.0 represent not only the cutting edge of AMS technology, but also its continuing evolution toward fulfilling our overall vision of providing a holistic, end-to-end content management solution that provides for all labelling requirements on a regulatory, marketing or packaging level. ” “ Working with our product development team, I look forward not only to expanding and improving on the existing performance of the system, but also to introducing new and innovative functionality that will allow our clients take advantage of the technological revolution taking place in content management ,” Mrs Ivory concluded. About GLAMS For Life Science organisations, managing artwork packaging and marcom workflows is complex, time consuming and prone to error. With labelling and artwork playing such a critical role in the regulatory mandated communication of medicinal formulation, usage instruction, safety and efficacy data and patient information, mistakes can happen! Involving thousands of people across multiple locations, keeping track of all artwork and labelling tasks, approvals and project timelines is a very challenging and costly exercise. Mistakes cause severe delays in product market launches, full product recalls and, in the worst cases, adverse patient reactions. To mitigate these risks, it is imperative that Life Science organisations have a fully validated and auditable artwork management system in place – a system that simplifies the packaging artwork labelling and regulatory processes and improves productivity. Designed and developed specifically for the Life Science industry Perigord’s GLAMS solution has one key differentiator from its AMS competitors – GLAMS is exclusively designed and developed for the Life Science industry. This means that every aspect of the solution, from the base architecture to each function, has a context that resonates with our Life Science partners. This makes the solution footprint more compact, simpler to understand, faster to validate and quicker to implement when compared with generic non-Life Science AMS solutions. Implementing GLAMS will have an immediate positive impact on your business, with measurable benefits far outweighing the cost of ownership. GLAMS is the only Artwork Management System dedicated to the sector and specifically designed to help reduce risk, speed up artwork turnaround times, remove duplication of effort, and deliver greater business value. Available from May 2021 onwards – for further insights and technical information, as well as learning new ways to boost business productivity, efficiency, and profitability: Request a demo at: www.glams.com/sign-up/request-demo/ For more information contact your GLAMS Account Manager or: Mark Ennis Global Business Development Director mark.ennis@perigord-as.com Steven Gaffney Business Development Executive steven.gaffney@perigord-as.com Perigord Life Science Solutions Unit 1, Lyncon Court IDA Business & Technology Park Snugborough Road, Blanchardstown Dublin, D15 N283, Ireland mediarelations@perigord-as.com Tel: +353 1 440 3222 www.GLAMS.com
11 Feb, 2021
We are delighted to announce the launch of our new Independent Packaging & Labelling Inspection Service . Over the years, we have worked with a number of organisations providing artwork production and coordination services around the globe. Increasingly we have been requested to support more and more of the proofing and inspection tasks companies such as yours need to carry out, these include a range of critical checks across the product packaging life cycle, be they: Pre-Artwork Checks Production Artwork Checks Braille Confirmation Barcode Review Technical Artwork Revisions Text Content Verification Printer Proofing As a result of this demand, we have set up a specific independent team to carry out these inspections of organisations' artwork – no matter the source. To learn more about this new service, please click below:
Social Media Feed
Corporate News

08 Mar, 2022
Today on this International Women's Day we'd like to put the spotlight on some very impressive and talented Women in Perigord. This important date on the calendar marks the liberation of women and gives them equal rights. 'Here's to strong women, may we know them, may we be them, may we raise them.'

20 May, 2021
Join us and your industry peers for our latest Webinar "How the power of a connected AMS and automated proofreading solution will improve the efficiencies of your process and increase your speed to market" on-demand now where experts from Dr. Pfleger Arzneimittel GmbH, Perigord and Schlafender Hase will share their experience and demonstrate how having an AMS system with integrated digital comparison functionality will increase your speed to market, reduce the risk of product recall, and provide a full end-to-end audit trail of all approvals.

10 May, 2021
As industry leaders and key sponsors at the Pharma Packaging & Labelling V-Forum on 26-27 May 2021 , we look forward to meeting with other pharma packaging supply chain professionals and reviewing the challenges and opportunities that the ever changing marketing dynamics are creating for our industry. As the pharmaceutical sector evolves, we will explore a number of key challenges that continue to beset the industry and add complexity in the artwork and labelling processes. Our COO, Global Software Division, Suzanne Ivory and Global Business Development Director, Mark Ennis will be in attendance. We hope you can join Suzanne who will be speaking on day 2 on these topics, providing a look into the future and outlining the solutions available to help pharmaceutical organisations navigate the challenges ahead.

05 May, 2021
Dublin, Ireland – May 05, 2021: Deep search capabilities and extensive integration options amongst suite of enhancements for life science-specific artwork management system GLAMS 5 Perigord announces the release of GLAMS 5.0 , the latest update to its industry-leading AMS platform. Developed to the specific needs of the Life Science industry, this latest release boasts an expanded suite of both new and improved features, as well as greatly improved performance. Perigord Data’s Chief Operations Officer, Suzanne Ivory explained, “GLAMS 5’s new deep search capabilities break down potential impediments to getting products to market, and allows users to create deep and wide-ranging searches of all their artwork data, including querying active text on any uploaded PDFs, making it possible to gather a huge amount of rich, context-filled information quickly, easily, and efficiently . ” With its history of innovation and close cooperation with some of the world’s biggest pharmaceutical companies, Perigord’s development team has added several new features to GLAMS to greatly increase the usability and productivity of the platform for its users. These include: Advanced search capabilities Enhanced built-in proofing and reporting tools Configurable document stamping Extensive integration enhancements Multilingual versions Performance improvements “ Listening to our customers and leveraging our own experience, we have spent several months refining the GLAMS platform , ” explained Paul Leamy, Group CTO and Head of Product Innovation at Perigord Data . “ We have added new functionality and increased the processing speed across our product suite. Not only do the new features offer our clients a more holistic experience when managing their artwork, GLAMS 5.0 will greatly improve the speed, usability, scalability, and efficiency of the artwork and labelling process across all phases of the packaging and labelling supply chain for our clients .” GLAMS is the only artwork management system built specifically for the exacting requirements of the Life Science industry and the release of GLAMS 5 is a further step toward cementing Perigord’s reputation as the go-to AMS solution provider in the sector. “ It is Perigord’s extensive expertise and experience that enables us to develop solutions that streamline the approval process and other aspects of artwork and labelling workflows, essentially removing the bottlenecks that delay the packaging development process ,” commented Perigord Data’s Director of Client Services, Sam Cole . Introducing GLAMS 5’s new features: Advanced search capabilities – Leveraging the power of OCR technology, the scope of GLAMS 5’s capabilities have increased massively, allowing users to search the text of supporting or approval documents. Searches have also been made faster and more intuitive, enhancing clients’ capacity to access artwork tasks quicker, create complex, ad hoc queries conveniently, and deliver even greater efficiency to the process. Enhancements to integrated proofing and reporting tools – Clients can seamlessly integrate with the newest versions of InformaIT, GlobalVision, TVT and other leading comparison tools on the market as well as enjoying improvements to the GLAMS 3D visualisation functionality. These enhancements also provide customers with the added ability to autosave reports from third-party applications directly into GLAMS jobs. Configurable document stamping – This extension to the existing GLAMS stamping functionality allows multiple approver stamps to be shown in a table on a separate page inserted into an approved artwork PDF. This improvement also allows for the inclusion of pagination on stamping activities, making quality assurance processes more configurable, more efficient, and providing greater traceability in offline, printed artwork and graphic files. Approver stamps are clearer and more easily located and viewed. Extensive integration upgrades – Updates to the GLAMS API layer deliver improved connectivity to external systems and make GLAMS the most integration-ready AMS on the market. This will enable users to send and receive data from a variety of sources including ERP systems like SAP or Oracle, as well as Regulatory Information, Documents Management, Change Control, and PLM systems, unleashing a huge range of digital capabilities. Multilingual versions – The GLAMS user interface can now be tailored to clients’ language preferences. Pre-configured, multi-lingual options now include Japanese and Mandarin, with the facility to add additional language packs as required. Performance improvements – Perigord’s continued focus on under-the-hood investment has led to a raft of improvements that speed up the loading of data in grids and drop-down values, allowing for faster job scheduling, quicker search replies and more efficient data export. These performance improvements deliver quicker response times, enhancing the user experience. “ With the new functionality and system improvements included in GLAMS 5.0, I am confident that GLAMS is the best Life Science artwork management system on the market ,” said Perigord Data’s COO, Suzanne Ivory . “ The improvements incorporated into GLAMS 5.0 represent not only the cutting edge of AMS technology, but also its continuing evolution toward fulfilling our overall vision of providing a holistic, end-to-end content management solution that provides for all labelling requirements on a regulatory, marketing or packaging level. ” “ Working with our product development team, I look forward not only to expanding and improving on the existing performance of the system, but also to introducing new and innovative functionality that will allow our clients take advantage of the technological revolution taking place in content management ,” Mrs Ivory concluded. About GLAMS For Life Science organisations, managing artwork packaging and marcom workflows is complex, time consuming and prone to error. With labelling and artwork playing such a critical role in the regulatory mandated communication of medicinal formulation, usage instruction, safety and efficacy data and patient information, mistakes can happen! Involving thousands of people across multiple locations, keeping track of all artwork and labelling tasks, approvals and project timelines is a very challenging and costly exercise. Mistakes cause severe delays in product market launches, full product recalls and, in the worst cases, adverse patient reactions. To mitigate these risks, it is imperative that Life Science organisations have a fully validated and auditable artwork management system in place – a system that simplifies the packaging artwork labelling and regulatory processes and improves productivity. Designed and developed specifically for the Life Science industry Perigord’s GLAMS solution has one key differentiator from its AMS competitors – GLAMS is exclusively designed and developed for the Life Science industry. This means that every aspect of the solution, from the base architecture to each function, has a context that resonates with our Life Science partners. This makes the solution footprint more compact, simpler to understand, faster to validate and quicker to implement when compared with generic non-Life Science AMS solutions. Implementing GLAMS will have an immediate positive impact on your business, with measurable benefits far outweighing the cost of ownership. GLAMS is the only Artwork Management System dedicated to the sector and specifically designed to help reduce risk, speed up artwork turnaround times, remove duplication of effort, and deliver greater business value. Available from May 2021 onwards – for further insights and technical information, as well as learning new ways to boost business productivity, efficiency, and profitability: Request a demo at: www.glams.com/sign-up/request-demo/ For more information contact your GLAMS Account Manager or: Mark Ennis Global Business Development Director mark.ennis@perigord-as.com Steven Gaffney Business Development Executive steven.gaffney@perigord-as.com Perigord Life Science Solutions Unit 1, Lyncon Court IDA Business & Technology Park Snugborough Road, Blanchardstown Dublin, D15 N283, Ireland mediarelations@perigord-as.com Tel: +353 1 440 3222 www.GLAMS.com

11 Feb, 2021
We are delighted to announce the launch of our new Independent Packaging & Labelling Inspection Service . Over the years, we have worked with a number of organisations providing artwork production and coordination services around the globe. Increasingly we have been requested to support more and more of the proofing and inspection tasks companies such as yours need to carry out, these include a range of critical checks across the product packaging life cycle, be they: Pre-Artwork Checks Production Artwork Checks Braille Confirmation Barcode Review Technical Artwork Revisions Text Content Verification Printer Proofing As a result of this demand, we have set up a specific independent team to carry out these inspections of organisations' artwork – no matter the source. To learn more about this new service, please click below:
Social Media Feed
Perigord Life Science Solutions A Tech Mahindra Company
Receive Perigord news and updates, and agree to the Privacy Notice by signing-up:
© 2024
Perigord Life Science Solutions